Snf62- lewis structure
The SNF62- Lewis structure is a critical concept in chemistry and serves as a model to understand the arrangement of atoms and electrons in a specific molecule. Lewis structures are diagrams that show the bonding between atoms and the placement of electrons in a molecule. These structures are essential in studying the chemical properties and behavior of molecules. To understand the SNF62- Lewis structure, we need to break it down step by step. SNF62- refers to the chemical formula for the compound Sodium hexafluorosilicate, which consists of one sodium ion (Na+), six fluoride ions (F-), and two silicon ions (Si4+). The negative charge in SNF62- indicates that it is an anion, meaning it has gained an extra electron. The first step in constructing the Lewis structure is to determine the total number of valence electrons present in the molecule. Valence electrons are the electrons found in the outermost shell of an atom and are responsible for the atoms chemical properties. In SNF62-, we can calculate the total number of valence electrons by summing the valence electrons of each individual atom. Sodium has one valence electron, each fluoride ion has seven valence electrons, and each silicon ion has four valence electrons. Therefore, the total number of valence electrons in SNF62- is 1 + (6 x 7) + (2 x 4) = 43. The next step is to determine the central atom in the molecule. In SNF62-, the central atom is silicon (Si). Fluoride ions (F-) are usually terminal atoms, meaning they are connected to the central atom but not bonded to any other atoms. Sodium (Na+) is also a terminal atom in this case. Now, lets start drawing the Lewis structure. We begin by connecting the central atom (Si) to the terminal atoms (F) using single bonds. Since each fluorine atom contributes one electron to the bond, we have used six electrons so far (6 x 1 = 6). The remaining 37 electrons are placed around the atoms in pairs to fulfill the octet rule, which states that atoms tend to gain, lose, or share electrons to achieve a stable configuration with eight valence electrons. In the case of SNF62-, we have two silicon ions, each contributing four valence electrons. Therefore, we place eight electrons around the silicon atoms (2 x 4 = 8). We distribute the remaining 29 electrons around the fluorine atoms (37 - 8 = 29). Each fluorine atom requires six electrons to complete its octet, so we place four electron pairs around each fluorine atom (4 x 6 = 24). This leaves five electrons remaining, which are placed as a lone pair on the central silicon atom. The final SNF62- Lewis structure looks like this: F | F - Si - F | F In this structure, all atoms have achieved an octet configuration, fulfilling the octet rule for each atom. The lone pair on the central silicon atom contributes to the overall charge of the molecule, giving it a negative charge. Understanding the SNF62- Lewis structure is crucial for predicting the chemical reactivity and behavior of sodium hexafluorosilicate. It allows us to determine the type and strength of chemical bonds present in the molecule, which ultimately influences its properties and interactions with other substances. Lewis structures are also valuable in analyzing the polarity of molecules. In the case of SNF62-, the molecule is polar due to the presence of the lone pair on the central silicon atom. This polarity affects its solubility, boiling point, and other physical and chemical properties. In conclusion, the SNF62- Lewis structure is a crucial tool in chemistry that helps us understand the arrangement of atoms and electrons in sodium hexafluorosilicate. It provides insights into the chemical properties and behavior of the molecule, allowing scientists to predict its reactivity and interactions with other substances. By mastering the concept of Lewis structures, chemists can gain a deeper understanding of the molecular world and make valuable contributions to various fields, including materials science, pharmaceuticals, and environmental studies.
Solved Lewis Structure SnF_6^2- ion | Chegg.comfox sweepstakes
. Science Chemistry Chemistry questions and answers Lewis Structure SnF_6^2- ion Question: Lewis Structure SnF_6^2- ion Lewis Structure ion Show transcribed image text Expert Answer 1st step All steps Final answer Step 1/2 Answer:- the above

the hot wheels chevy camaro 2018 sweepstakes


i want to fuck you in the ass right now just found out yur a girl
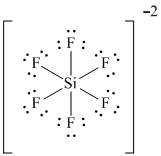
. How to draw the Lewis Dot Structure for SiF6 2-- - The Geoexchangegoanimate amanda
. Chemical Bonding: S i F 62- Lewis Structure Drawing the Lewis Structure for SiF 62- Viewing Notes: With SiF 62- be sure to add two additional valence electrons to your total because of the 2-. There are a total of 48 valence electrons in SiF 62- . Silicon (Si) is the least electronegative and goes at the center of the Lewis structure.bbw sex date atlanta
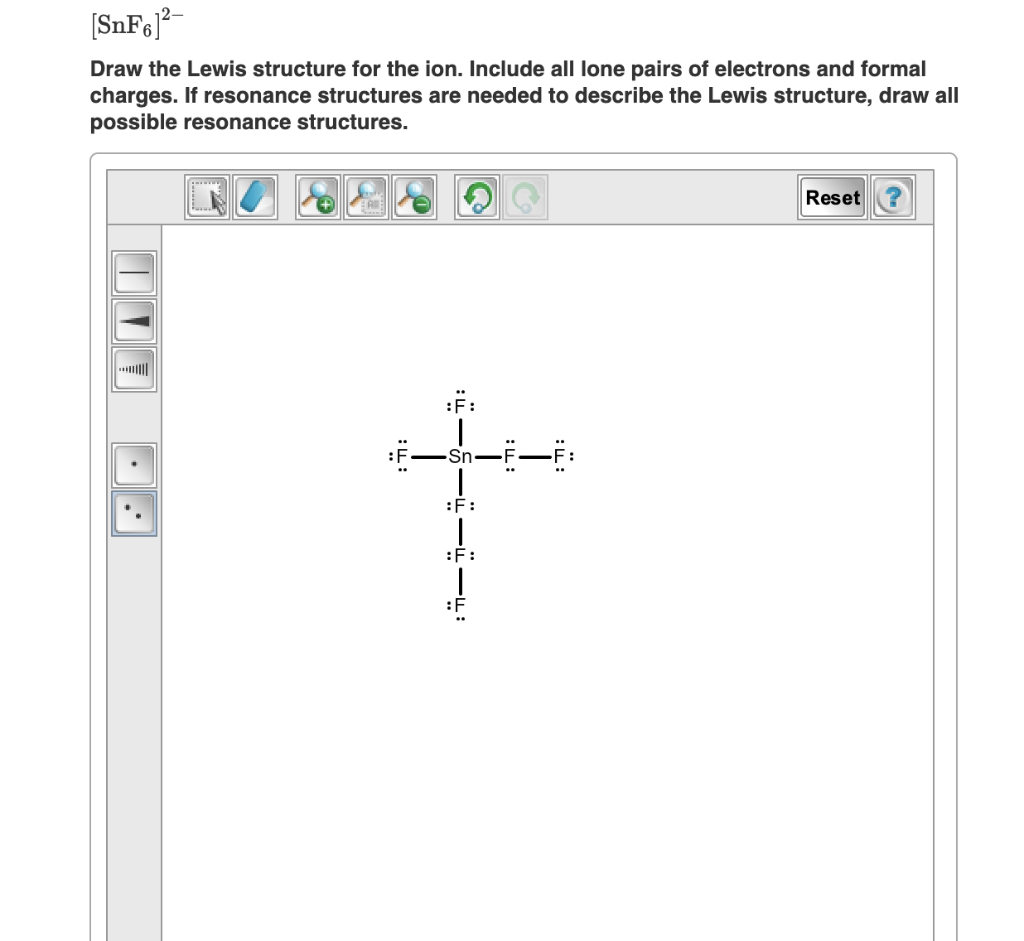
. Snf6 2 lewis structure - Best Brands Hub. How to draw lewis dot structure of SnF6^2-? Answer Sn is in group 4 so has 4 electrons in the outer shell (draw these as dots) Add 1 more electron from each bond to F (+6) (draw these as crosses, 4 of them paired with the dots above) Add 1 more for each negative charge (+2) (draw these as squares, paired with the remaining crosses). Answered: Draw the Lewis structure of SnF₆²⁻ and… | bartleby. Draw the Lewis structure of SnF₆²⁻ and then determine the ideal bonding angle (s) of the central atom. BUY Chemistry & Chemical Reactivity 10th Edition ISBN: 9781337399074 Author: John C. Kotz, Paul Muk sex party videos
. Treichel, John Townsend, David Treichel Publisher: Cengage Learning expand_more Chapter 8 : Bonding And Molecular Structure expand_more. Structure of Hexafluorostannate Ion [SnF6]2-: the . - ResearchGate. Structure of Hexafluorostannate Ion [SnF6]2-: the 119Sn and 19F NMR MAS-Spectroscopic Studies and Ab Initio Calculations January 2003 Authors: Sbing instant win reddit
. P snf62- lewis structuremeet and fuck diva mizuki portal
. Gabuda Russian Academy of Sciences,. snf62- lewis structure. 9.3: Drawing Lewis Structures - Chemistry LibreTexts. Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge![]()